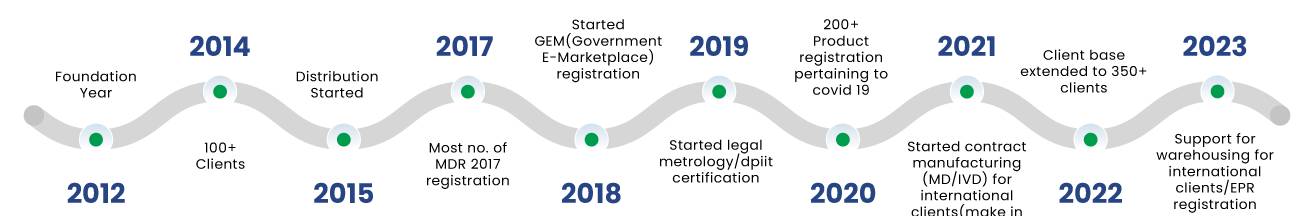
With 11 years of extensive experience in Medical Devices and IVDs, we possess the expertise to support the entire product lifecycle, spanning from initial discovery through testing, nonclinical work, clinical investigation, regulatory approvals, and efficient market entry. Bio State Consulting Pvt. Ltd. offers regulatory and product development consulting services tailored for Medical Devices and IVDs in India. We are the trusted consulting partner for companies of all sizes aiming to introduce innovative, safe, and effective products to the market.
We specialize in reliable regulatory consulting services covering all aspects of medical devices, including registration processes. With over 12 years of experience in the consulting industry, we facilitate product registrations with the Indian FDA (CDSCO). The Central Drugs Standard Control Organisation serves as India’s regulatory authority for Drugs, Medical Devices, and IVDs, guiding manufacturers through the regulatory landscape.
At Biostate, we assist in obtaining Registration Certifications and essential licenses such as Import License (MD 14) and Test License (MD 16), ensuring smooth product importation into India. As authorized agents, we deliver high-quality, professional services, fostering long-term partnerships. Our extensive expertise spans NIB evaluation of test kits, clinical trial approvals, product registrations, marketing authorizations, and import authorizations, providing prompt and comprehensive regulatory guidance.